Development of factor VIII (FVIII) inhibitor is a serious complication of factor replacement therapy observed in about 15-30% of severe hemophilia A (HA) patients. Inhibitors interfere with the treatment by neutralizing intravenously injected FVIII. Although bleeding rate is not increased in severe HA patients who developed the inhibitor, the inhibitors reduce the efficacy of factor replacement therapy during bleeding episodes and in consequence accelerate the progression of hemophilic arthropathy. In Poland, prophylaxis in hemophilia with inhibitors has been introduced in 2016, and therefore many HA patients with inhibitor still suffer from particularly severe arthropathy, and have a reduced quality of life.
The aim of the study was to evaluate the musculoskeletal health and quality of life in patients with severe hemophilia A with inhibitor before initiation of personalized physiotherapy and after 2 years of the personalized physiotherapeutic program.
The study was performed in 24 persons with severe HA with inhibitor (mean age 42.1 years, median age 42 years) receiving prophylaxis regimen with aPCC 85 ± 15 IU/kg 3 times a week. At the beginning of the project, the patients were examined by hematologist and physiotherapist. Joint condition was evaluated using the Hemophilia Joint Health Score (HJHS) and magnetic resonance imaging of the most affected joint. Quality of life was evaluated using EQ-5D scale (EuroQoL). aPCC dosing schedule in context of rehabilitation program was adjusted by the hematologist.
Each patient was enrolled to a personalized exercise program developed based on age, general condition, stage of arthropathy, date of the last bleeding episode, and a history of physiotherapy. The program consisted of weekly 60 to 90 minutes rehabilitation sessions at home, and participated in four 5-day rehabilitation camps with 5 hours of rehabilitation sessions a day organized in Poznan after 6, 12, 18 and 24 months of the program. All patients were examined by hematologist, and the rehabilitation was provided only to the patients on regular prophylaxis with aPCC provided that a dose of aPCC was always administered 1 hour before the initiation of rehabilitation session.
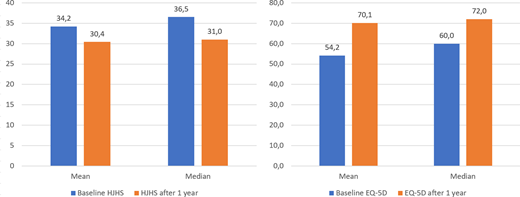
HJHS varied among the participants, with mean value at baseline 34.2 (median 36.5). Baseline mean EQ-5D score was 54.2 (median 60). Evaluation at month 12 was performed in 18 participants (2 patients withdrawn from the program, and 4 patients could not participate in the evaluation due to COVID-19 pandemics).
The results obtained at month 12 show a significant improving the quality of life and musculoskeletal health of patients with HA after combined prophylaxis and personalized rehabilitation. Mean HJHS decreased from 34.2 to 30.4 points (median from 36.5 to 31 points). An average 3.8 point decrease confirms positive effects of long-term aPCC prophylaxis with personalized, regular rehabilitation. Increase in mean EQ-5D score from 54.2 to 70.1 (median from 60 to 72) show enhancing patients' mood resulted from the improved health status and regained capability to conduct more normal lifestyle.
After the first year of the project we can conclude that regular prophylaxis with aPCC and personalized, once-a-week rehabilitation program positively influence joint status and quality of life of adult HA patients with inhibitor complicated with significant arthropathy. Regular rehabilitation may slow down the progression of arthropathy due to the choice of the exercise program appropriate for each patient. These positive effects justify the continuation of the project in patients with hemophilia A with inhibitor.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.